Grantee Research Project Results
2008 Progress Report: An Integrated Computational Framework for the Interpretation of Organophosphorus Pesticide Biomarkers
EPA Grant Number: R833451Title: An Integrated Computational Framework for the Interpretation of Organophosphorus Pesticide Biomarkers
Investigators: Reisfeld, Brad , Lyons, Michael A. , Chambers, Janice E. , Mayeno, Arthur N. , Yang, Raymond S.H.
Institution: Colorado State University , Mississippi State University
EPA Project Officer: Hahn, Intaek
Project Period: October 1, 2007 through September 30, 2010
Project Period Covered by this Report: October 1, 2007 through September 30,2008
Project Amount: $748,582
RFA: Interpretation of Biomarkers Using Physiologically Based Pharmacokinetic Modeling (2006) RFA Text | Recipients Lists
Research Category: Hazardous Substance Research Centers , Pesticides , Human Health
Objective:
Although various biomarkers have been used to assess exposure to and poisoning from organophosphorus (OP) pesticides/insecticides, the complexity of OP absorption, distribution, metabolism, and elimination, especially for mixtures of these chemicals, warrants integration of computational modeling tools with the biomarker data for more accurate quantitation and assessment of actual whole body exposures and target tissue dosimetry. The objective of this project is to create a computer-assisted framework to aid in the identification, characterization, and understanding of biomarkers resulting from human exposure to mixtures of OP insecticides, using chlorpyrifos and diazinon as the initial test compounds. The framework will use existing human biomarker data, along with information about population and exposure variability and uncertainty, to reconstruct absorbed dose and exposure scenarios, as well as to predict levels of biomarkers resulting from known exposures to one or multiple OP insecticides.
Modeling Program: The goals of the modeling portions of this project that are being conducted at Colorado State University (CSU) have not changed. The objective remains to create a computer-assisted framework to aid in the identification, characterization, and understanding of biomarkers resulting from human exposure to mixtures of OP insecticides, using CPF and DZN as the initial test compounds. Work to date has focused on several different areas: (i) data mining, (ii) constructing the OP insecticide PBPK models, (iii) improving the simulation core of the software framework, and (iv) developing a graphical user interface for the framework.
Progress Summary:
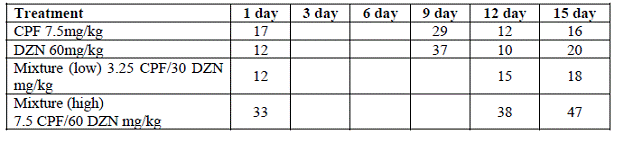
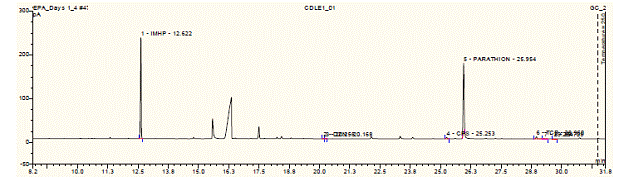
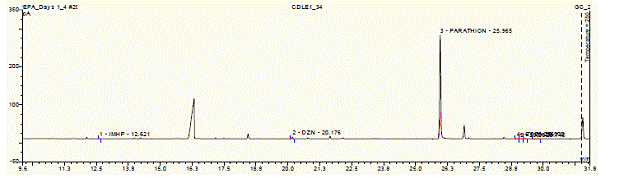
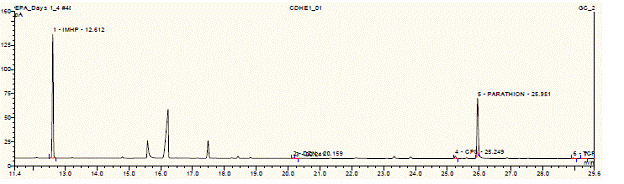

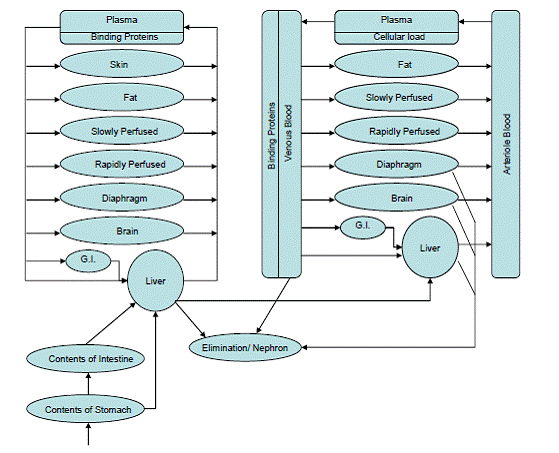
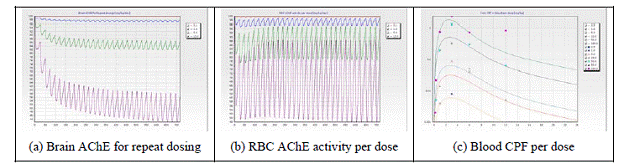
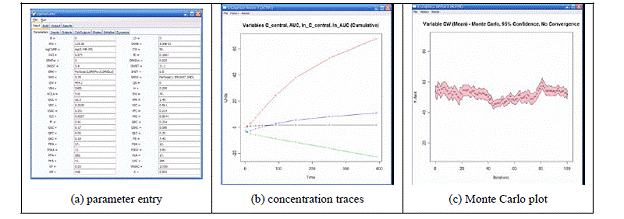
Future Activities:
Journal Articles on this Report : 2 Displayed | Download in RIS Format
| Other project views: | All 3 publications | 2 publications in selected types | All 2 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Lyons MA, Yang RS, Mayeno AN, Reisfeld B. Computational toxicology of chloroform: reverse dosimetry using Bayesian inference, Markov chain Monte Carlo simulation, and human biomonitoring data. Environmental Health Perspectives 2008;116(8):1040-1046. |
R833451 (2008) |
|
|
|
Reisfeld B, Ivy JH, Lyons MA, Wright JM, Rogers JL, Mayeno AN. DoseSim: a tool for pharmacokinetic/pharmacodynamic analysis and dose reconstruction. Bioinformatics 2013;29(3):400-401. |
R833451 (2008) |
Exit Exit Exit |
Progress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
